Abstract
Introduction: Adults with sickle cell disease (SCD) suffer from vaso-occlusive pain episodes (VOEs) for which they often receive care in infusion clinics (ICs). Typically, 80% of patients treated for VOE are discharged home but it is not clear how long it takes to recover to baseline functioning after an acute visit.1 Recent clinical trials have struggled to identify practical and useful outcomes for novel therapies that decrease the length of VOE or pain during hospitalization. As many patients with VOE are seen and treated in an IC and discharged home, identifying potential endpoints for novel therapies in this site of care are needed. The purpose of this study was to measure time to recovery for two weeks post an IC visit for management of VOE and to identify patient characteristics that predict a return visit for pain management within that time period.
Methods: A convenience sample of adults with SCD (≥18YO) were consented to and enrolled into this IRB-approved study during a visit to the IC for VOE. Medical records of all subjects were reviewed to collect data on the number of acute visits, hospitalizations, use of disease-modifying therapies, use of pain medications, baseline laboratory data, and comorbidities. Data was collected on the date VOE pain started, pain scores before and after treatment, and analgesic management. Baseline surveys were collected on the day of the visit and patients were sent follow-up surveys after discharge electronically through REDCap on post-discharge days 2,4,6,8,10,12,14. Participants were asked to rate their pain on a 0-10 Visual Analog Scale. The PROMIS pain interference and Fatigue scales were used to assess pain impact and the Subjective Opioid Withdrawal Scale (SOWS) was used to assess for signs of opioid withdrawal. Return to baseline functioning was estimated by asking participants at each contact post-visit to rate their current level of functioning in activities of daily living on a scale of 0-100, with 100 being their baseline level of function. A similar scale was used to measure the resolution of pain, with the anchor being "back to your usual, non-crisis, level of daily pain." Subjects were encouraged to complete surveys through text message reminders and were compensated with a fifty-dollar gift card if all surveys were completed. Cox proportional hazards modeling was used to examine predictors of time to a return acute care visit within 14 days of the index visit.
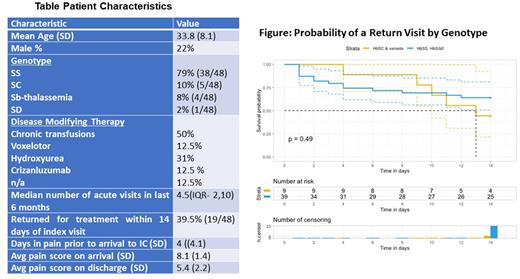
Results: Table 1 shows the characteristics of the 48 patients enrolled in the study. Despite multiple text/email reminders, few patients completed all the surveys (8/48). Thirty-two out of 48 people completed initial surveys. Of the 17 patients who completed a day 2 or later SOWS score 7 had moderate or severe withdrawal symptoms and of those 3 had a return visit within 14 days while 5/10 of those with mild scores had a return visit. Nineteen out of 48 patients returned for treatment within 14 days. Controlling for pain at discharge, sex, and genotype only the number of acute visits in the six months prior to the index visit was associated with a return visit in 14 days (HR 1.31, 95% CI: 1.13 1.53). The figure shows the probability of a return visit over time by genotype. The median number of visits the entire cohort had in the six months prior to the index visit was 4.5 (IQR: 2,10) however, in those patients that returned for treatment within 14 days the median number of visits in the last six months was 8 (IQR: 5,11).
Conclusions: In this study of patients' experience post an acute visit for pain management at an IC, we identified that the number of acute visits in the six months prior to the index visit predicted a return visit within 14 days. It was challenging for patients to complete surveys after their acute visits suggesting that this was not a feasible way to collect these important patient-reported outcomes. With almost 40% of patients returning for an acute care visit within 14 days, decreasing the percentage of patients with return visits may be a potential outcome for trials of novel therapies to treat VOE in the IC setting.
1. Lanzkron S, Carroll CP, Hill P, David M, Paul N, Haywood C, Jr. Impact of a dedicated infusion clinic for acute management of adults with sickle cell pain crisis. Am J Hematol. 2015;90(5):376-380.
Disclosures
Lanzkron:HRSA: Research Funding; CSL-behring: Research Funding; Takeda: Research Funding; Novartis: Research Funding; GBT: Research Funding; Imara: Research Funding; Glycomimetics: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Current equity holder in private company; PCORI: Research Funding; Teva: Current equity holder in private company; Novo Nordisk: Other: Adjudication committee; bluebird bio: Other: Adjudication committee, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.